Tel:0513-81903181
Email:info@cop-nb.com
Address:Ningbo Petrochemical Economic and Technological Development Zone
Tel: 0513-81903181
Email: info@cop-nb.com
1. Product Introduction
The CCOP® HTM series products from Huanxiting feature high transparency, high moisture resistance, drug compatibility, high fracture resistance, low protein adsorption, sterilization compatibility, and halogen-free properties. They can be used for medical and biological purposes and are highly suitable for pre-filled syringes, vials, ampoules, intravenous infusion bags, barrier films, blister packaging, and other pharmaceutical packaging applications.
Series of products | HTM-700/1000/1300/1600

2. Application fields
The performance of the HTM series is highly matched with the core requirements of "safety, stability, and adaptability" for pharmaceutical packaging, and the specific application fields and adaptability logic are as follows:
Injection packaging: Pre-filled syringes, ampoules
Adaptation characteristics: low protein adsorption + drug compatibility, avoiding active loss of biologics (monoclonal antibodies, peptides); high tear resistance reducing the risk of damage during transportation.


Liquid preparation packaging:Injection bottles, intravenous infusion bags
Adaptation characteristics: high moisture-proofness + no layering, extending the shelf life of the drug solution; high transparency for easy observation of the drug solution state.

Special formulation packaging:Dried freeze packaging
Adaptation characteristics: low-temperature tensile elongation rate adapts to freeze-drying process; ultra-low permeability blocks external pollutants.
Generic drug packaging:Blister packaging, carton bottles
Adaptation characteristics: excellent molding performance supports customized design; sterilization compatibility adapts to a variety of sterilization processes.
3. Product characteristics
The core features of the HTM series are designed around "pharmaceutical safety and formulation protection", as follows:
High transparency:
Parameters: glass-like high light transmission, low haze;
Pharmaceutical value: It is convenient for medical staff to intuitively check the clarity of the liquid and whether there is precipitation, enhancing the safety of medication.
High moisture resistance:
Parameter: extremely low gas/water permeability;
Pharmaceutical value: effectively blocks the moisture / oxygen from the outside, extending the shelf life of easily oxidized, hydrolyzed drugs.
Drug compatibility:
Parameters: resistant to acid, alkali, alcohol and organic solvents, suitable for a wide range of pH values;
Pharmaceutical value: to avoid interaction between the packaging material and the drug solution, ensuring the purity and stability of the drug.
High toughness:
Parameters: excellent high impact strength + low temperature tensile elongation;
Pharmaceutical value: Reduce the damage rate during transportation and use; meet the low-temperature process requirements of lyophilized preparations.
Low protein adsorption:
Parameters: low surface energy + hydrophobicity, extremely low protein binding rate;
Pharmaceutical value: Adapted for the packaging of monoclonal antibodies, recombinant proteins and other biologics, avoiding the loss of drug active ingredients.
Ultra-high purity:
Parameters: Residual metal content below the limit of detection, no processing aids;
Pharmaceutical value: Reduce the risk of leachables/dissolutes, comply with the high purity requirements of pharmaceutical packaging materials.
Sterility compatibility:
Parameters: support EO (ethylene oxide), Gamma (gamma rays), EB (electron beam), Steam (steam) sterilization;
Pharmaceutical value: Adapt to the sterilization process of different formulations, enhance the flexibility of packaging process.
Lightweight characteristics:
Parameters: the weight ratio is only 1/2.5 of glass;
Pharmaceutical value: Reduce logistics and storage costs, and enhance the convenience of packaging operations.
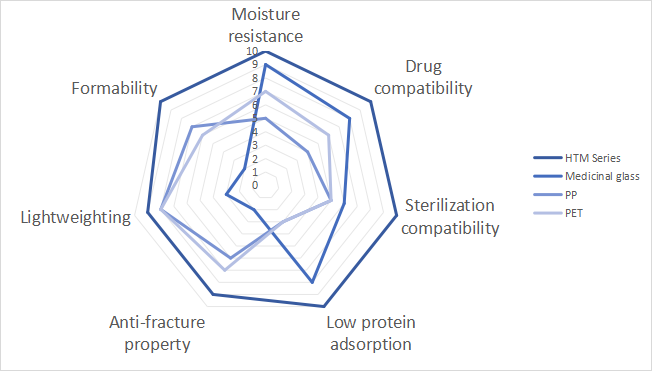
4. Comparison of the advantages of other materials
With pharmaceutical packaging materials commonly used (pharmaceutical glass, PP, PET) as a reference: